
购物车0
产品总数:61446

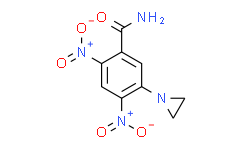
| 商品编号 | 规格 | 价格 | 会员价 | 是否有货 | 数量 |
|---|---|---|---|---|---|
| PL03471-5mg | 5mg | ¥1285.00 | 请登录 |
|
|
| PL03471-10mg | 10mg | ¥2009.00 | 请登录 |
|
|
| PL03471-50mg | 50mg | 询价 | 询价 |
|
|
| PL03471-100mg | 100mg | 询价 | 询价 |
|
|
| PL03471-10mM*1mLinDMSO | 10mM*1mLinDMSO | ¥2229.00 | 请登录 |
|
 扫码关注公众号
扫码关注公众号